Healthcare-associated infections (HAIs) account for an additional $28 to $33 billion in healthcare costs each year. Approximately 1.7 million infections and 99,000 associated deaths occurred in 2002.The Centers for Disease Control's (CDC) identifies the problem of antimicrobial resistance as an emerging threat to world health; cites the cost of hospital-acquired infections "caused by just six common kinds of resistant bacteria" to be "at least $1.3 billion per year."
ICET has identified a clear need to develop bio polymeric interfacial surfaces which
a) Prevent the adherence of bacteria.
b) Prevent calcium and magnesium salt deposits on the material.
 ICET has addressed these and developed highly lubricious and efficacious surface coating formulations with antimicrobial properties. Such formulations are coatable on silicone, polyurethane, PVC, metals and latex devices.
ICET has addressed these and developed highly lubricious and efficacious surface coating formulations with antimicrobial properties. Such formulations are coatable on silicone, polyurethane, PVC, metals and latex devices.
 The coating process is simple such as dip coating and a few milligrams of the coating could provide active protection. Ready to coat or custom formulations can be provided.
The coating process is simple such as dip coating and a few milligrams of the coating could provide active protection. Ready to coat or custom formulations can be provided.
- Clinically poor performance of existing anti-microbial catheters on the market is now well recognized and there is a dire need for infection control urinary catheters.
- With the end of reimbursement for costs associated with Catheter associated urinary Tract Infection, (CAUTI)hospitals are eager to prevent or reduce their incidence of thiscondition, and are examining their urinary catheter management practices. Evidence-based guidelines are available, but are not universally followed.
- ICET developed the TIC Foley system with a coated Foley catheter connected to an accessory and fully qualified the product with the FDA for an IDE and FDA guidance on functionality and in vitro performance :2008-2010.
 ICET catheter vs. Competitor silver based catheter- note heavy biofilm on the competition catheter; recent clinical trials on existing commercial antimicrobial Foley catheters have not shown clinical promise- but the ICET coated urinary catheter system has shown a promising tend as evidenced by the recent pilot human clinical data in comparison with a competitor catheter
ICET catheter vs. Competitor silver based catheter- note heavy biofilm on the competition catheter; recent clinical trials on existing commercial antimicrobial Foley catheters have not shown clinical promise- but the ICET coated urinary catheter system has shown a promising tend as evidenced by the recent pilot human clinical data in comparison with a competitor catheter
http://www.clinicaltrials.gov/ct2/show/NCT01681511?term=ICET&rank=)
Antimicrobial and Therapeutics Releasing Platform Technology
Compounding Technologies ICET can provide its custom antimicrobial formulations for compounding with certain medical resins for dry extrusion processes. The ICET active formula could be compounded with medical or consumer grade resins and extruded to a desired shape. The temperature limitations for this process are 300 F.
Currently an extruded antimicrobial catheter accessory device, Foley Guard connected to the ICET antimicrobial catheter was tested in a clinical trial. This has been fully qualified for biocompatibility and functionality and ICET has obtained an Investigational Device Exemption form the FDA.
ICET can provide its custom antimicrobial formulations for compounding with certain medical resins for dry extrusion processes. The ICET active formula could be compounded with medical or consumer grade resins and extruded to a desired shape. The temperature limitations for this process are 300 F.
Currently an extruded antimicrobial catheter accessory device, Foley Guard connected to the ICET antimicrobial catheter was tested in a clinical trial. This has been fully qualified for biocompatibility and functionality and ICET has obtained an Investigational Device Exemption form the FDA.
ICET Link : Antimicrobial Technology for Food and Beverage Packaging
ICET formulations coated catheter with repeated challenges of microbes provide potent reductions in microbial growth over a period of several days.
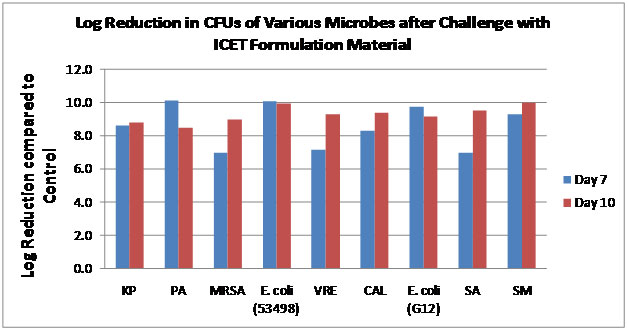
Graph depicts the log reduction in microbial growth as compared to uncoated controls. ICET formulations are challenged with 106 CFUs/mL twice during over a period of 10 days. The two challenges occur before the 7th day. Plating results indicate at least 99.999% reduction in microbial growth as compared to the control (not shown) for days 7 (blue bars) and 10 (red bars). Organisms tested from left to right, Klebsiellapneumoniae, Pseudomonasaeruginosa, Staphylococcus aureus (MRSA), Escherichia coli, Enterococcus faecalis (VRE), Candida albicans, Staphylococcus aureus, and Serratiamarcescens. All organisms are urological clinical isolate.
Log Reduction of Microbes in the Contact Solution Compared to a Control and after Seven Days Incubation.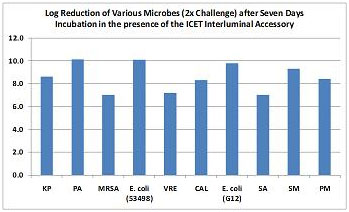
Assay Criteria : At least 4 logs reduction (99.99%) of growth (CFUs/mL) in the Foleyguard sample to the control. Organisms from left to right: Klebsiellapneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus(MRSA), Escherchia coli, Enterococcus faecalis(VRE), Candida albicans,E. coli, S. aureus, Serratiamarcesscens, Proteus mirabilis.
- Four US Patents secured from 1999- 2007; one international including India.
- Catheter accessory first generation prototype made : 2002-2003
- Pilot production & clinical finished by 2005
ICET completed an initial Human clinical trials on an add-on antimicrobialdevice accessory only connected to a commercial the Foley catheter. Conducted at U. Wisconsin Hospitals under the direction of Dennis Maki M.D, a world renowned Infectious disease specialist.
(Published in Urology Times, 2005; Neoscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC™) Washington D.C 2005.
ShanthaSarangapani *, Katherine Cavedon, David Gage Journal of Biomedical Materials Research, Volume 29 Issue 10, Pages 1185 - 1191,1995
Therefore ICET Foley Catheter coatings were developed &qualified for biocompatibility per ISO 10993.
Unique, Proprietary Silver Accentuating Chemistry
- ICET synergistic formulation provides a triple defense mechanism against bacterial and fungal microbial biofilms and prevents calcium and magnesium deposits.
- No known microbial resistance for silver, safe history, easier FDA approval process.
- Maintaining wetting with lubrication allows sustained diffusion of silver species and synergistic agents.
- Adjustable sustained long term release that can be incorporated into polymeric coatings and extrudable plastics.
ICET Advantage :
Proprietary silver chemistry with sustained release for superior antimicrobial performance.
- Key advantage, safety history of silver accepted by FDA.
- Antiseptic like chlrohexidine, triclosan, Nitrofurazone cause mucosal irritation and hypersensitivity; antibiotics coatings last only for short periods and the risk for the selection of resistance exists.
- ICET is currently an R&D company that is transitioning into a manufacturing company.
- ICET works with several manufacturing partners. They provide vendor services to ICET.
Antimicrobial coated catheters are an attractive solution for Infection prevention in short term use (less than 7 days) and to date the only two major silver based Foley catheters in the market have not shown much clinical promise, though silver is an excellent biocide, with a good safety history, used in various medical devices for successful infection control (FDA CDRH data base). Rather than cast doubt on the efficacy of silver as a biocide based on the one type of Foley catheter type evaluated, many clinicians and the FDA believe that NOT all silver products are the same; the efficacy depends on the continuous delivery mechanism of silver from the coating on the surface and that clinical trials of new silver-coated urological products remain critically important. Our product TIC is not "yet another silver catheter" but may prove to be a cut above the rest.
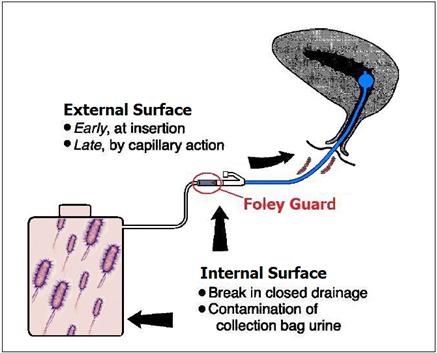 The ICET- TIC closed drainage system with microbial entry routes is shown. An FDA approved Silicone Foley Catheter is coated on internal and external surfaces including the balloon that release soluble silver in a sustained manner for up to 14 days; the accessory (Foley Guard TM) connected to the catheter outlet is outside the body, that releases copper and silver in a soluble form, is designed to block ascending infections as colonized urine collection systems constitute a reservoir of resistant organisms in hospitals.
The ICET- TIC closed drainage system with microbial entry routes is shown. An FDA approved Silicone Foley Catheter is coated on internal and external surfaces including the balloon that release soluble silver in a sustained manner for up to 14 days; the accessory (Foley Guard TM) connected to the catheter outlet is outside the body, that releases copper and silver in a soluble form, is designed to block ascending infections as colonized urine collection systems constitute a reservoir of resistant organisms in hospitals.



